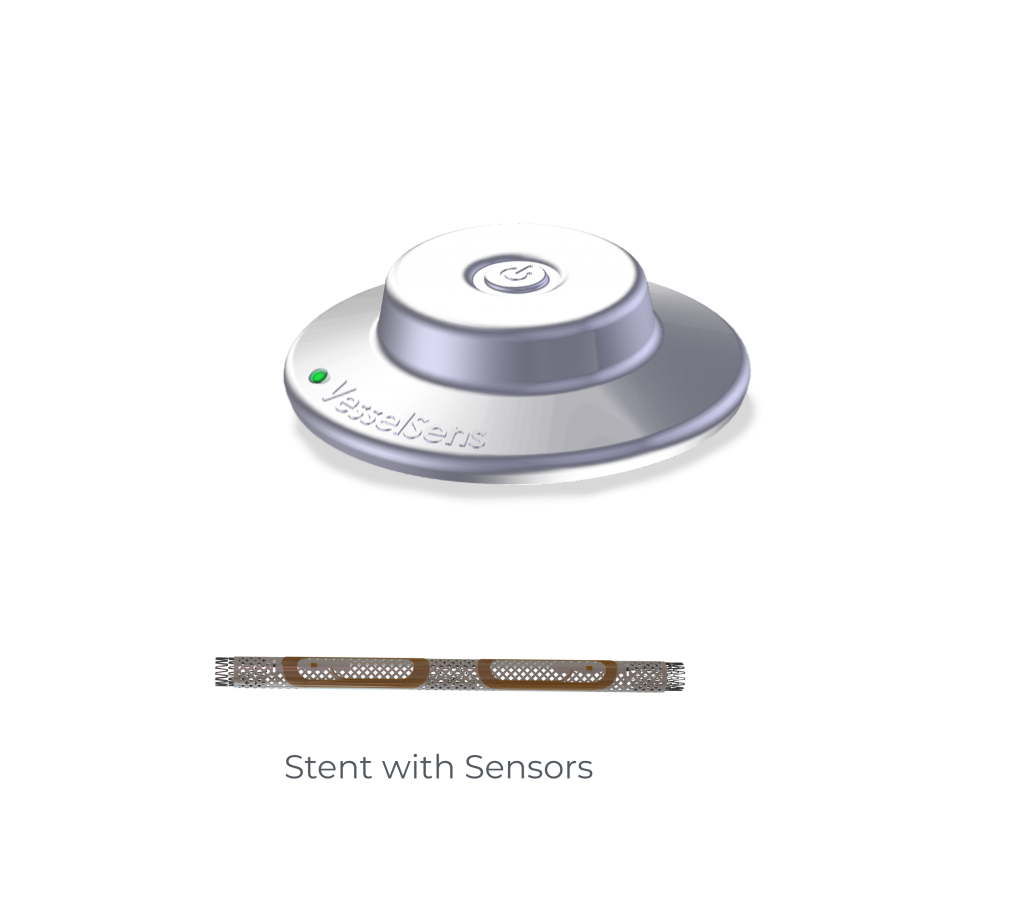
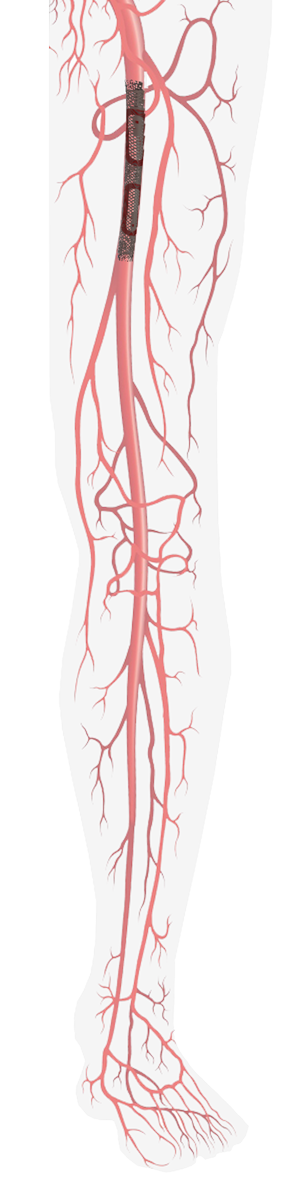
... Several Advantages
vs. State-of-the-Art
- Measurements in short intervals
- Early prediction of clogging
- Easy handling, precise and fast diagnosis

For Physicians
- Determination of trends and correlation with further vital parameters is possible
- The restenosis treatment may be induced in an earlier stage
- Success of therapy is detectable and can be monitored

For Patients
- Improves life comfort and sense of security
- Early detection
- Reduction of time-consuming check-ups, e.g. physical performance tests, ultrasonic testing